Abstract
Background: Post-transplant lymphoproliferative disorder (PTLD) is a rare complication of solid organ or hematopoietic transplantation and is composed of a heterogeneous group of lymphoid disorders ranging from plasmacytic hyperplasia to aggressive lymphomas. Several factors such as age, performance status, lactate dehydrogenase, presence of B-symptoms, and disease stage have been identified as poor prognostic factors in PTLD. Extranodal disease (END) is present in the majority (50-80%) of patient (pts) at the time of diagnosis and has been shown to a be predictor of overall survival in this patient population; in particular, the presence of disease involvement in the central nervous system (CNS) or bone marrow (BM) has been shown to be poor prognostic factor. In this single center, retrospective analysis we examine the impact of END on outcomes in pts with PTLD.
Methods: Pts with a diagnosis of PTLD were identified by Electronic Medical Records database query. Inclusion criteria were age ≥ 18 years at the time of diagnosis, confirmation of PTLD by internal pathology review, primary diagnosis from 2008-2018, and receipt of therapy and surveillance care at Northwestern University in Chicago, Illinois, USA. Exploratory univariate analyses were performed using Kaplan-Meier estimates with 95% confidence intervals and log-rank p-values for time-to-event outcomes; significance was set at p < 0.05. Response to treatment and disease progression were classified per provider-specific interpretations of clinical data as assessed by patient chart review.
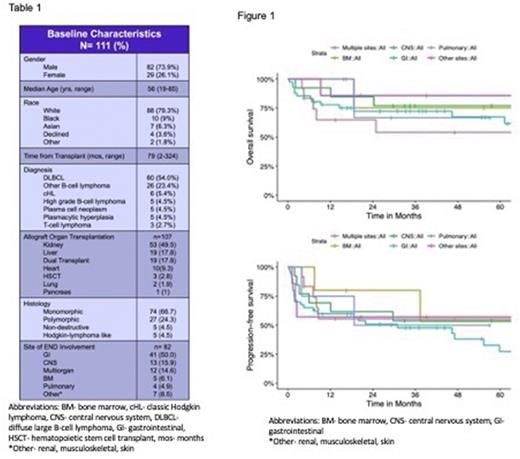
Results: A total of 182 pts with a diagnosis of PTLD were identified by database query and 111 pts met inclusion criteria. Of the 111 pts included in this analysis, 82 pts (73.9%) were male with a median age at diagnosis of 56 years (range 19-85) (table 1). The median time from transplant to diagnosis was 79 months (range 2-324). For all pts, the five-year overall-survival (OS) and progression-free survival (PFS) were 70.7% and 49.7%, respectively. Eighty-two pts (73.9%) had END at the time of diagnosis. There was no difference in 5yr OS between pts with END and those without END (69.5% vs 73.3%, p=0.56). A trend towards improved PFS was noted in patients without END compared to those patients with END; however, this difference was not statistically significant (70.6% vs 42.4%, p=0.09). Out of the 82 pts with END, 12 pts (14.6%) had multiple-site disease involvement, 41 pts (50%) had only gastrointestinal (GI) involvement, 13 pts (15.9%) CNS, 5 pts (6.1%), 4 pts (4.9%) pulmonary involvement, and 7 pts (8.5%) had other organ site involvement (renal, musculoskeletal, or oropharyngeal). There was no difference in 5yr OS and PFS between patients with single organ involvement of END vs multiple-site END at time of diagnosis. There was no difference in OS in patient with PTLD when stratified out by specific site involvement; however, patients with END disease involvement of GI tract had significantly inferior PFS than those patients without GI (27.3% vs 60.6%, p=0.05) (Figure 1). There was no difference in PFS across the other sites of END involvement.
Conclusions: This study represents one of the largest retrospective cohorts of PTLD reported in the literature. We show that overall, the presence of END does not correlate to worse PFS or OS in patients with PTLD. The presence of GI involvement at the time of diagnosis may correlate to worse PFS. No additional specific site of organ involvement, including presence of BM or CNS disease, correlated to worse overall prognosis in this patient population.
Disclosures
Friedewald:CSL Behring: Research Funding; Eurofins - Transplant Genomics: Consultancy; Regeneron: Research Funding; Hansa BioPharma: Research Funding; Veloxis: Research Funding; Eurofins - Viracor: Research Funding. Winter:Merck & Co., Inc.: Honoraria, Research Funding; Cellectis: Other: for Spouse, to the University of Chicago, Research Funding; Servier: Consultancy, Other: For Spouse; CVS/Caremark: Consultancy, Other: For Spouse; Astellas: Other: For Spouse, to University of Chicago, Research Funding; Rafael: Other: For Spouse, to University of Chicago, Research Funding; Forty Seven/Gilead: Other: For Spouse, to University of Chicago, Research Funding; Novartis: Consultancy, Other: for Spouse, to the University of Chicago, Research Funding; Daiichi Sankyo: Other: for Spouse, to the University of Chicago, Research Funding. Gordon:Zylem: Current equity holder in private company, Current equity holder in publicly-traded company, Patents & Royalties: Patent on nanoparticles for lymphoma therapy; BMS: Research Funding; Janssen: Other: DSMB; Ono Pharmaceuticals: Consultancy. Karmali:Eusa: Consultancy; Karyopharm: Consultancy; Kite: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau; Takeda: Research Funding; BMS/Celgene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Other: Advisory Board; Genentech/Roche: Consultancy, Other: Advisory Board; Calithera: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; AstraZeneca: Other: Advisory Board, Speakers Bureau; Morphosys/Incyte: Consultancy, Other: Advisory Board, Speakers Bureau; BeiGene: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau. Ma:Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Loxo: Research Funding; Bristol Myers Squibb: Consultancy; TG Therapeutics: Consultancy, Research Funding; Juno: Research Funding; BeiGene: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding. Pro:Seattle Genetics: Honoraria. Moreira:CTI BioPharma: Consultancy; Ingenio Rx: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal